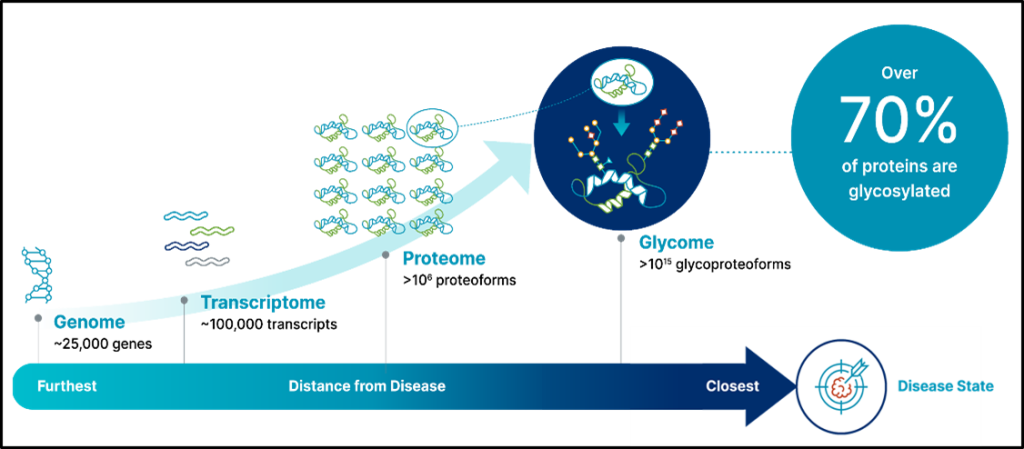
Glycoproteomics, a subset of proteomics, investigates proteins that contain glycans (oligosaccharides) within their structure, studying both their configurations and functions. As protein glycosylation involves intricate, multi-step enzymatic processes, the oligosaccharides attached to proteins exhibit highly diverse structures. Besides gathering general glycosylation details about glycoproteins, comprehending the variability in glycosylation sites and the oligosaccharides’ makeup is crucial for a comprehensive grasp of glycoproteins’ biological functions.
The examination of glycopeptides, derived from breaking down proteins into peptide units using proteases, is conducted at the glycopeptide level. Research at this level can concentrate on intricate glycoproteomes. Insights gained at the glycopeptide level encompass details regarding the glycosylation sites’ locations, the structure of the attached glycans, and the sequences of peptides. In contrast, the glycan level focuses solely on the structures of glycans within a given glycoproteome. Clinical approaches in glycoproteomics, using blood and tissue samples, enable site-specific investigations, potentially leading to the identification of new glycopeptide-based biomarkers crucial for early disease diagnosis.
Our objective is to identify novel candidates for glycopeptide-based biomarkers and devise an analytical method for the early detection of diseases.