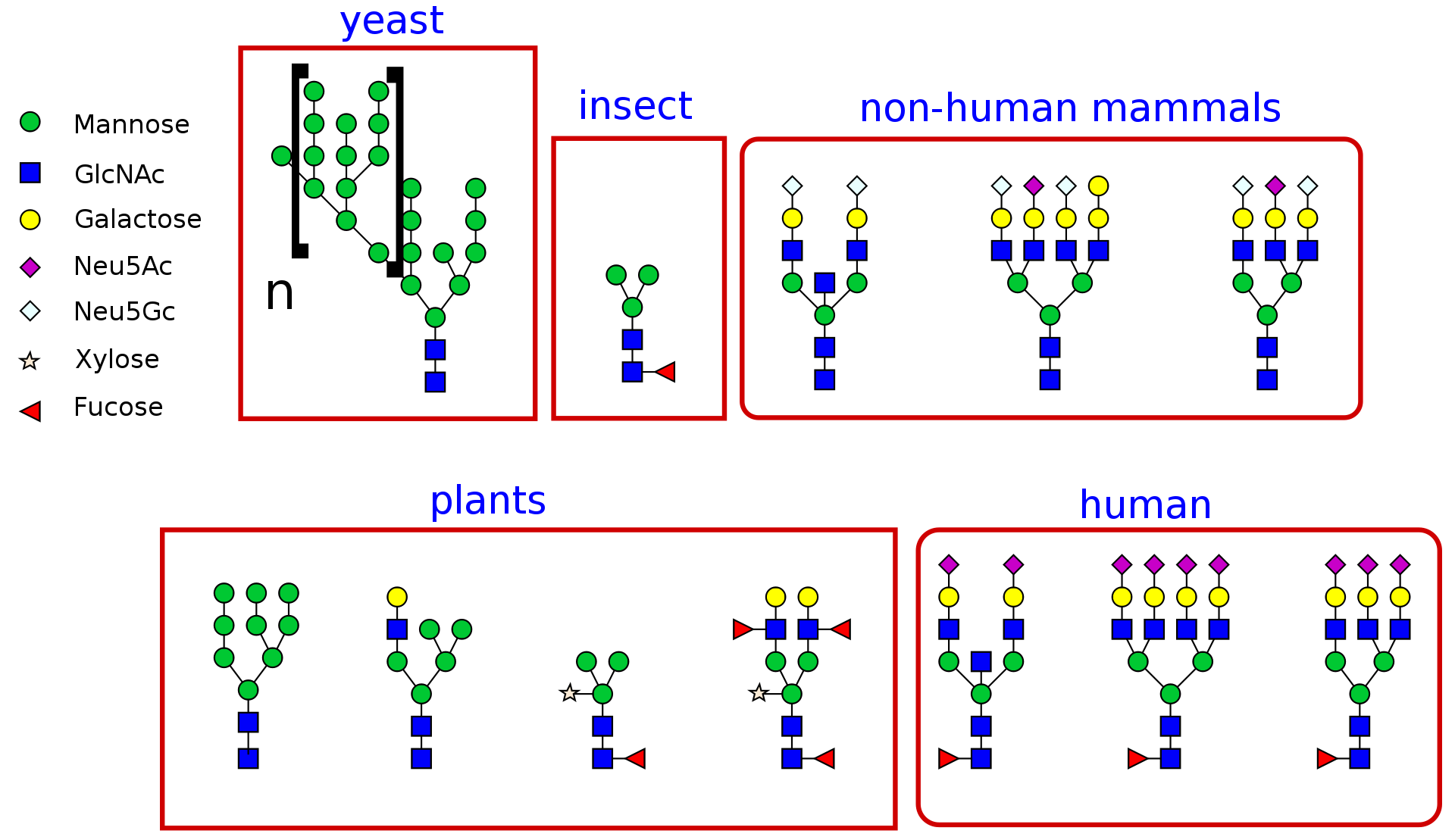
Glycosylation stands as a significant post-translational modification process. It’s estimated that glycosylation occurs in approximately 50% of the entire proteome within living organisms. This process involves a series of enzymatic reactions catalyzing the attachment of oligosaccharide units. In simple terms, these units are connected to hydroxyl groups on serine and threonine amino acids through O-glycosidic bonds or to asparagine amino acids via N-glycosidic bonds, referred to as O-linked and N-linked glycosylation, respectively. Glycosylation plays a pivotal role in altering the functional properties of proteins, with glycosylated proteins serving critical functions. Glycans that bind to proteins play a vital role in governing crucial processes within living organisms. For instance, thyroid glycoproteins are involved in hormone biosynthesis in the thyroid gland. Detecting mutations occurring during glycosylation is of paramount importance, as abnormal glycosylation in protein structures has been linked to conditions such as cancer, genetic diseases, and immune system disorders. Therefore, to gain a comprehensive understanding of glycosylated proteins and their functions, it is essential to determine their structures using robust and dependable techniques.
Our goal is to utilize mass spectrometry methods to analyze and identify N- and O-glycosylation sites on significant glycoproteins.